As the first layer of physical and immunological defense, the skin serves multiple critical functions in the body. The robust extracellular matrix (ECM) of the skin is intrinsic to many of these defense abilities, but also makes skin cell isolation a more difficult and longer process than other tissues. The dermis and epidermis contain a dense webbed network of collagen, elastin, fibronectin, and other proteins which hold cells in place (1). This complicates detailed study of skin cells for immunophenotyping, immunotherapies, investigations of allergies, studies on inflammation, and other applications. However, once the ECM is disrupted, single cell suspensions can be analyzed by flow cytometry and single cell sequencing to research skin more deeply (2).
Skin tissue’s especially stiff architecture makes dissociation more difficult than other tissues (3). A commercially available enzymatic approach requires at least 17 hours, while in this experiment we show that the SimpleFlow™ Skin Dissociation protocol using Bulk Lateral Ultrasonic (BLU™) energy requires only 15 minutes to achieve a viable single cell suspension, while maintaining cells at a temperature below 6°C.
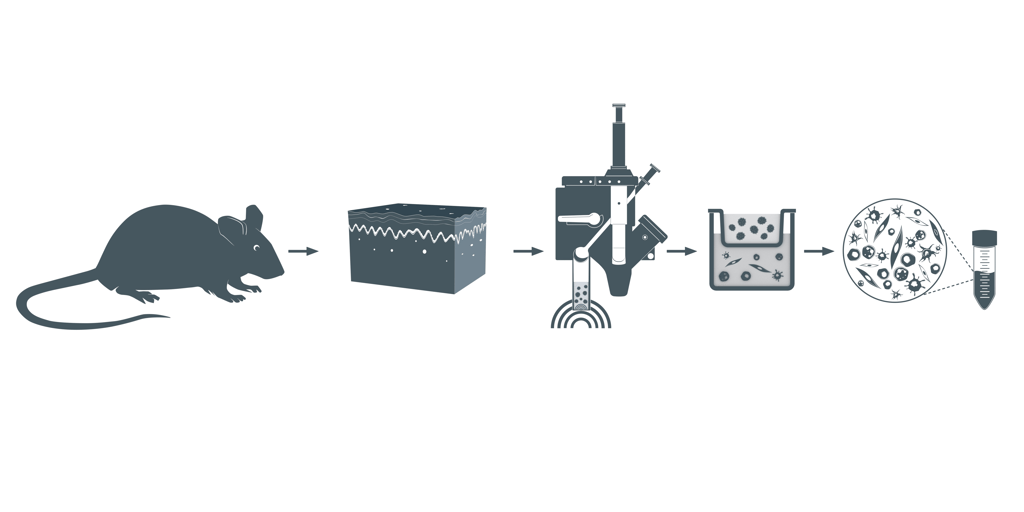 Figure 1. Visual representation of the SimpleFlow Skin Dissociation workflow. Following skin harvest, BLU energy dissociates single cell suspensions which are filtered to yield a viable single cell suspension.
Figure 1. Visual representation of the SimpleFlow Skin Dissociation workflow. Following skin harvest, BLU energy dissociates single cell suspensions which are filtered to yield a viable single cell suspension.
Trunk skin from one mouse was obtained from Antibody Solutions (Santa Clara, CA, USA) with hair, fat, and excess fibrous tissue removed. Skin was weighed and divided into ~130 mg aliquots (N=3). Skin was minced and ground in a mortar and pestle containing RPMI. Solid and partially ground tissue and media was transferred to the dissociation chamber of the SimpleFlow cartridge. The empty mortar was rinsed with additional RPMI, which was also added to the dissociation chamber. Samples were then dissociated with BLU energy before filtration and centrifugation. The cell pellet was resuspended, replicates were combined, and live and dead cell count data was collected.
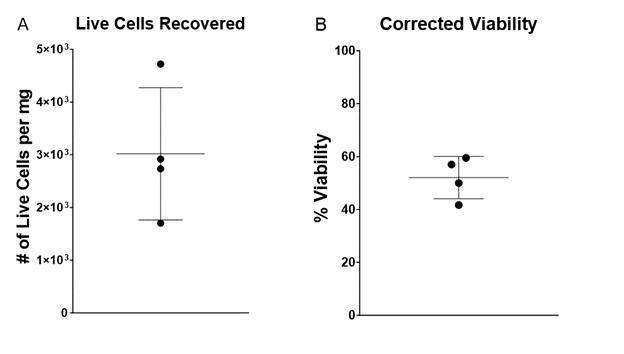
A count of live cells per milligram of tissue was measured using a hemacytometer with a Trypan Blue live/dead exclusion assay. Cell viability of four technical replicates (N = 4) was also measured using a Trypan Blue live/dead exclusion assay on the Cellometer® Spectrum (Cat. No. 8002632, Nexcelom, Lawrence, MA, USA). Cells with a diameter below 6 μm were excluded from corrected viability calculations (4).
This protocol employs a short 15-minute end-to-end dissociation workflow with BLU energy to recover an average of 3000 cells per milligram of tissue input. The ability to recover a population of live skin cells viable for downstream applications such as flow cytometry and single cell sequencing in only a few minutes can greatly accelerate the pace of research on the functionality and diseases of the skin.
In addition to the time-saving benefits for researchers, this 15-minute protocol addresses concerns raised by Burja et al. about the detrimental effects of stress factors such as mechanical damage, heated digestions, and long enzymatic digestions that may impact downstream analysis of dissociated skin cells (2). Utilizing gentle mechanical dissociation at temperatures below 6°C, the SimpleFlow Skin Dissociation protocol using BLU energy offers nearly real time results and may also preserve the health and diversity of dissociated cells for downstream analysis.
- Krieg T, Aumailley M. The extracellular matrix of the dermis: flexible structures with dynamic functions. Exp Dermatol. 2011;20(8):689–95.
- Burja B, Paul D, Tastanova A, Edalat SG, Gerber R, Houtman M, et al. An Optimized Tissue Dissociation Protocol for Single-Cell RNA Sequencing Analysis of Fresh and Cultured Human Skin Biopsies. Front Cell Dev Biol [Internet]. 2022 [cited 2023 Aug 30];10. Available from: https://www.frontiersin.org/articles/10.3389/fcell.2022.872688
- Jiang Y, Zhang H, Wang J, Liu Y, Luo T, Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol OncolJ Hematol Oncol. 2022 Mar 24;15(1):34.
- 9780521106818_excerpt.pdf [Internet]. [cited 2023 Oct 13]. Available from: https://assets.cambridge.org/97805211/06818/excerpt/9780521106818_excerpt.pdf
Interested in learning more?
Ask for a 20-minute seminar from a Cellsonics Scientist




.png?width=300&height=54&name=CellsonicsLogoWhite(2048%20x%20366%20px).png)