Tissue dissociation is a crucial sample preparation step that enables the isolation of specific cell populations for downstream applications such as single-cell RNA sequencing (scRNA-seq) or fluorescent activated cell sorting (FACS). Enzymatic tissue dissociation is a widely used method, but some researchers have concerns about the impact of enzymes on cell marker expression as outlined by Mattei et al (1) .
Here, we compare the use of a commercially available enzymatic tissue dissociation kit with the Bulk Lateral Ultrasonic (BLU) energy used in the SimpleFlowTM platform to isolate single cells from B16 melanoma mouse tumors in two independent experiments performed at Grand Valley State University through our academic collaboration with Dr. Kristin Renkema’s laboratory. BLU gently disaggregates solid tissue through acoustic energy without applying heat or enzymes. This fast, automated process dissociates tumors or tissue into a single cell suspension in as little as 3 minutes per sample while retaining the sample at a temperature of 6°C.
In brief, three (3) C57BL/6 mice in the first independent experiment (E1) and five (5) C57BL/6 mice in the second independent experiment (E2) were given subcutaneous injections of B16 melanoma cells. Tumor growth and mouse health were monitored regularly. When tumors reached an appropriate size, mice were euthanized, and tumors were removed and weighed. Isolated tumors were separated into two treatment groups, enzymatic and BLU dissociation. The enzymatic group was processed using a commercially available tumor dissociation kit employing enzymes and heat per the manufacturer’s protocol. The BLU group was dissociated via a manual mince step within the BLU cartridge and was subsequently pulsed with low levels of acoustic energy for 2 minutes. Samples from both groups were stained and analyzed via flow cytometry. Cell counts were determined with CountBright™ absolute counting beads (Invitrogen, C36950) and marker expression was evaluated in FlowJo (BD).
We first compared the number of cells isolated from mouse tumors using BLU to those isolated using the enzymatic method (Fig. 1A-B). In both experiments, cell count per milligram of tissue was not found to be statistically significantly different based on dissociation method.
Next, we looked at the subset of CD8+ T cells in each population. In both experiments, tumor samples isolated with BLU had significantly increased expression of the cytotoxic T cell marker, CD8 (E1: MFI = 807.7, E2: MFI = 1424), than those isolated using enzymatic approaches (E1: MFI = 103.7, E2: MFI = 781.4). As a result, the single cell population dissociated by BLU had a significantly higher number of CD8+ cells per milligram (Fig. 1C-D). This aligns with research done by Autengruber, et.al which shows diminished surface expression of CD8 on cells dissociated with Dispase (2).
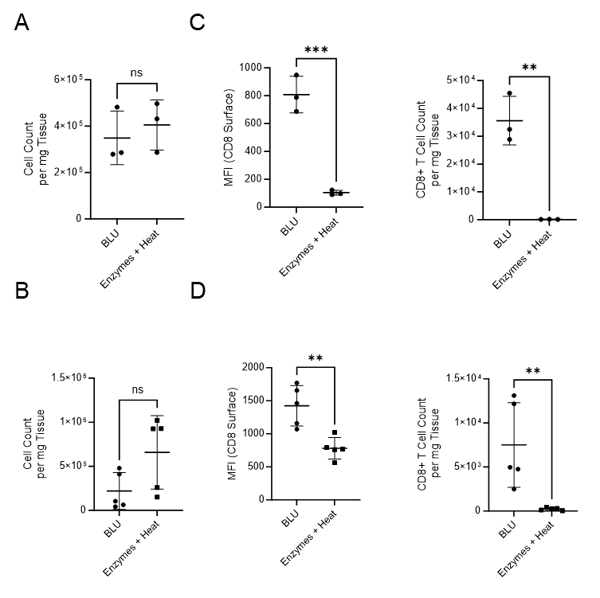
Fig. 1. Comparison of cell count per milligram of tissue, CD8 surface expression and CD8+ T cell count per mg of tissue following B16 melanoma tumor dissociation by enzymatic or BLU approaches. (A) E1 cell count per mg tissue, N = 3 biological replicates per group. (B) E2 cell count per mg tissue, N = 5 biological replicates per group. (C) E1 (N = 3) CD8 surface expression and CD8+ T cell subset counts per milligram of tissue. (D) E2 (N = 5) CD8 surface expression and CD8+ T cell subset counts per milligram of tissue. ns = not significant, * = p-value ≤ 0.05, ** = p-value ≤ 0.01.
We then compared the cell surface co-expression of other known T cell markers, CD3 and CD45, on our CD8+ T cell subset. Relative to CD8- CD4- tumor cells, we demonstrated that the CD8+ T cell populations isolated from BLU dissociation in E1 (Fig. 2) and E2 (Fig. 3) express CD3 and CD45. These data, in combination with the significant increase in the number of CD8+ T cells isolated from the BLU method of tumor dissociation, suggest that BLU can be used for a more effective CD8+ T cell dissociation from B16 melanoma tumors than is possible with current enzymatic methods.
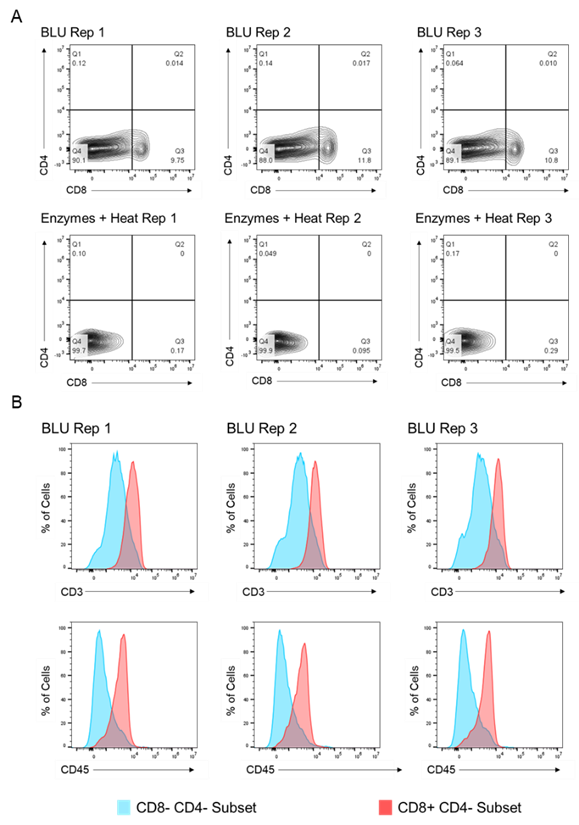
Fig. 2. Co-expression of CD45 and CD3 on CD8+ T cells isolated by BLU tissue dissociation in E1 (N = 3). (A) CD8+ T cell selection. (B) CD3 and CD45 co-expression on CD8+ T cell subsets. Note: Too few CD8+ T cells were isolated by enzymatic dissociation to enable a comparable assessment.
Here we demonstrate an alternative to enzymatic tissue dissociation, SimpleFlow, which uses Bulk Lateral Ultrasonic energy to gently dissociate cells from tissue. In this study, BLU effectively dissociated cells from mouse B16 melanoma tumors without the use of enzymes and while maintaining the sample at 6°C, enabling the isolation of a significantly larger CD8+ T cell population for downstream applications than was attainable by enzymatic approaches.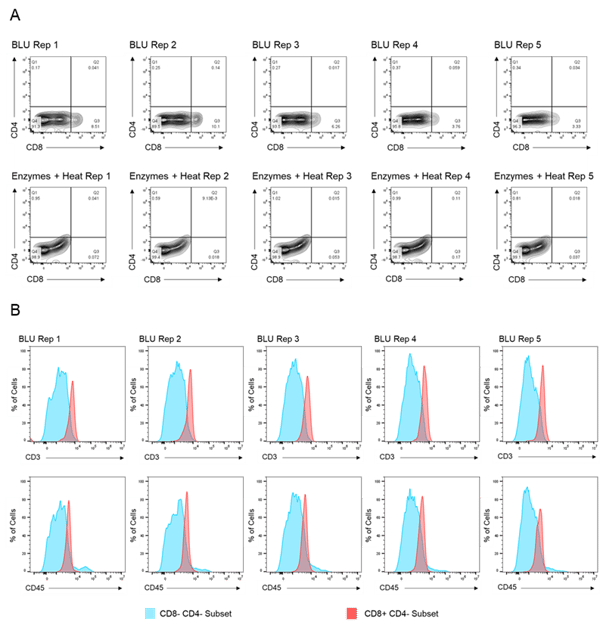
Fig. 3. Co-expression of CD45 and CD3 on CD8+ T cells isolated by BLU tissue dissociation in E2 (N = 5). (A) CD8+ T cell selection. (B) CD3 and CD45 co-expression on CD8+ T cell subsets. Note: Too few CD8+ T cells were isolated by enzymatic dissociation to enable a comparable assessment.
Interested in enhancing your cell analysis with improved tissue dissociation? Ask for a demo and we will bring the SimpleFlow to you. Your cells deserve the best.
1. Mattei D, Ivanov A, van Oostrum M, Pantelyushin S, Richetto J, Mueller F, Beffinger M, Schellhammer L, Vom Berg J, Wollscheid B, Beule D, Paolicelli RC, Meyer U. Enzymatic Dissociation Induces Transcriptional and Proteotype Bias in Brain Cell Populations. Int J Mol Sci. 2020 Oct 26;21(21):7944. doi: 10.3390/ijms21217944. PMID: 33114694; PMCID: PMC7663484.
2. Autengruber A, Gereke M, Hansen G, Hennig C, Bruder D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur J Microbiol Immunol (Bp). 2012 Jun;2(2):112-20. doi: 10.1556/EuJMI.2.2012.2.3. Epub 2012 Jun 13. PMID: 24672679; PMCID: PMC3956959.
Interested in learning more?
Ask for a 20-minute seminar from a Cellsonics Scientist




.png?width=300&height=54&name=CellsonicsLogoWhite(2048%20x%20366%20px).png)